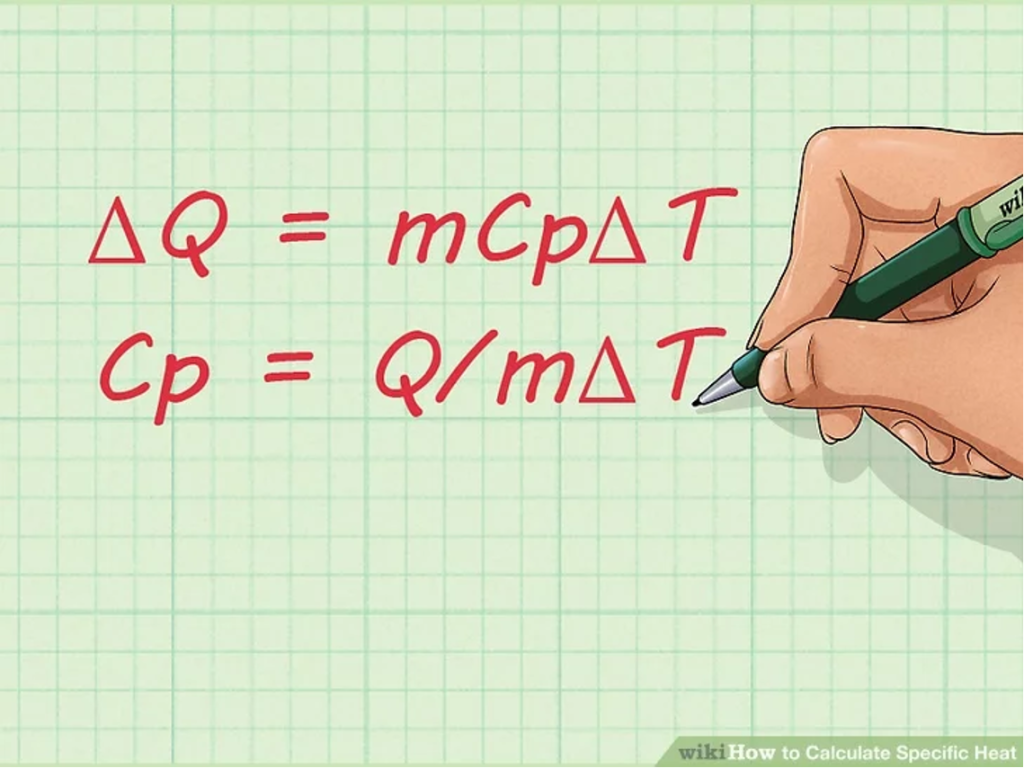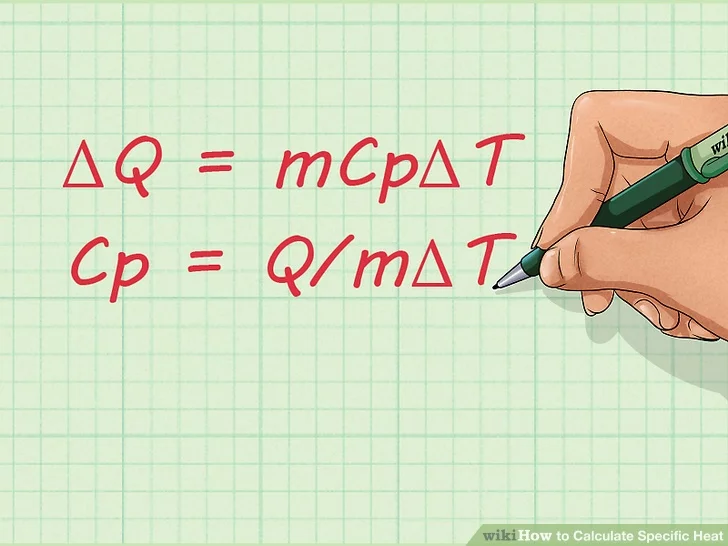How to Calculate Specific Heat
Specific heat is the amount of energy required to raise one gram of a pure substance by one degree Centigrade. The specific heat of a substance is dependent on both its molecular structure and its phase. The discovery of specific heat sparked the studies of thermodynamics, the study of energy conversion involving heat and the work of a system. Specific heat and thermodynamics are used extensively in chemistry, nuclear engineering, and aerodynamics, as well as in everyday life in the radiator and cooling system of a car. If you want to know how to calculate specific heat, just follow these steps.
1. Learn the Fundamentals.
1. Become familiar with the terms that are used for calculating specific heat. It’s important to be familiar with the terms that are used for calculating specific heat before you learn the formula for specific heat. You’ll need to know how to recognize the symbol for each term and to understand what it means. Here are the terms that are commonly used in the equation for calculating the specific heat of a substance:
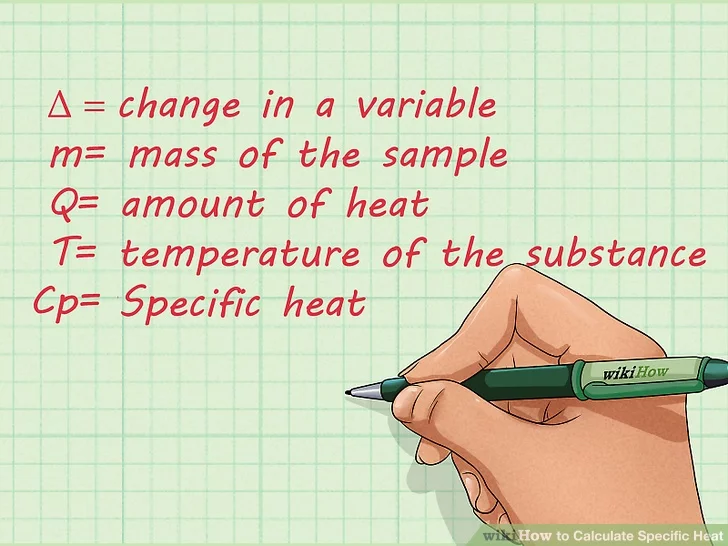
- Delta, or the “Δ” symbol, represents the change in a variable.
- For example, if your first temperature (T1) is 150ºC, and your second temperature (T2) is 20ºC, then ΔT, or the change in temperature, represents 150ºC – 20ºC, or 130ºC.
- The mass of the sample is represented by “m”.
- The amount of heat is represented by “Q”. The amount of heat is represented by “J”, or Joules.
- “T” is the temperature of the substance.
- Specific heat is represented by “Cp“.
2. Learn the equation for specific heat. Once you become familiar with the terms used for calculating specific heat, you should learn the equation for finding the specific heat of a substance. The formula is: Cp = Q/mΔT.
- You can manipulate this formula if you want to find the change in the amount of heat instead of the specific heat. Here’s what it would look like:
- ΔQ = mCpΔT
2. Apply the equations
1. Study the equation. First, you should look at the equation to get a sense of what you need to do to find the specific heat. Let’s look at this problem: Find the specific heat of 350 g of an unknown material when 34,700 Joules of heat are applied, and the temperature rises from 22ºC to 173ºC with no phase change.
2. List the known and unknown factors. Once you’re comfortable with the problem, you can write down each known and unknown variable to have a better sense of what you’re working with. Here’s how you do it:
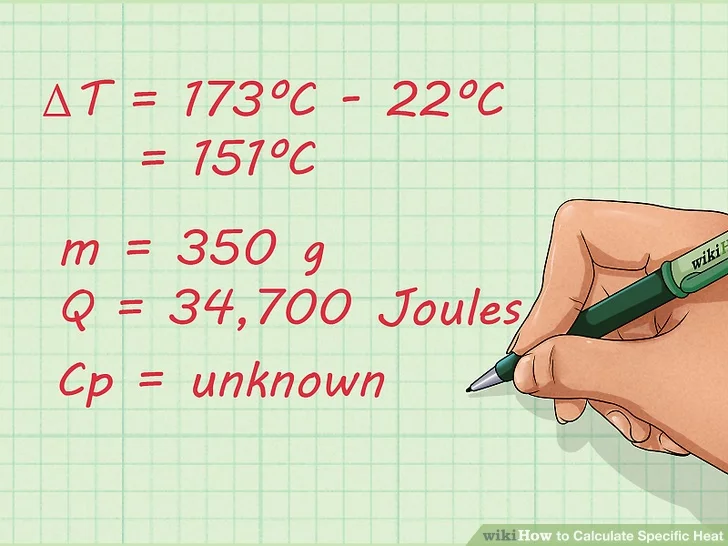
- m = 350 g
- Q = 34,700 Joules
- ΔT = 173ºC – 22ºC = 151ºC
- Cp = unknown
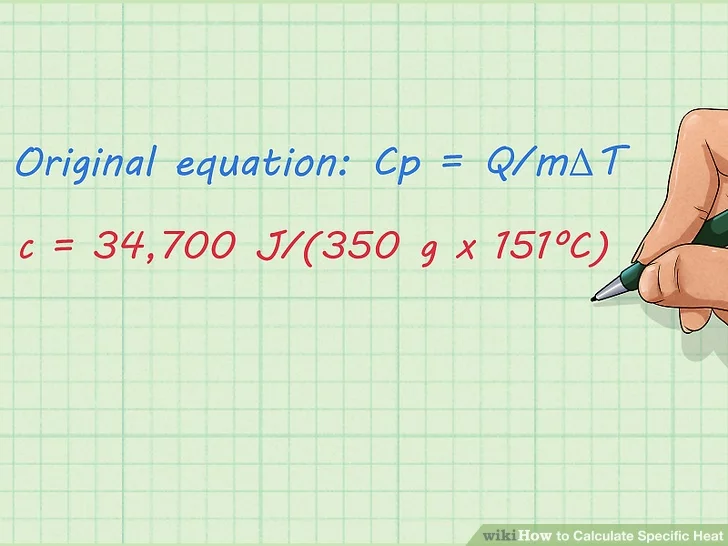
3. Plug the known factors into the equation. You know the value of everything except “Cpc”, so you should plug the rest of the factors into the original equation and solve for “Cp“, Here’s how you do it:
- Original equation: Cp = Q/mΔT
- c = 34,700 J/(350 g x 151ºC)
Calculate Specific Heat
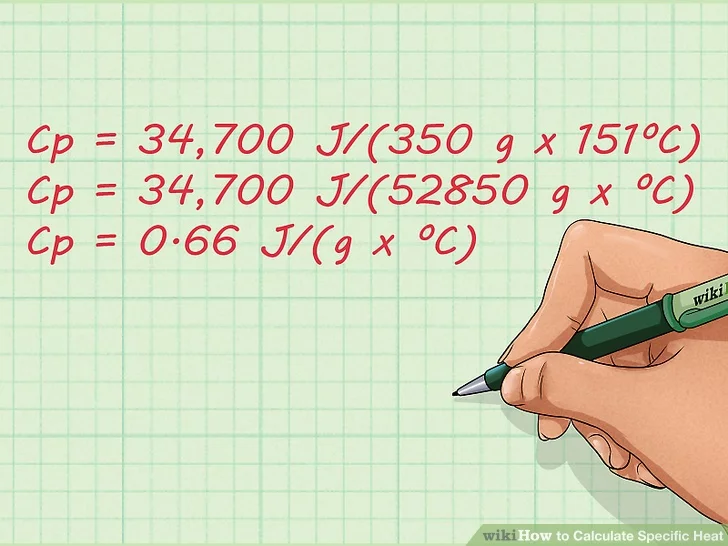
4. Solve the equation. Now that you’ve plugged the known factors into the equation, just do simple arithmetic to solve it.[5] The specific heat, or final answer, is 0.65657521286 J/(g x ºC).
- Cp = 34,700 J/(350 g x 151ºC)
- Cp = 34,700 J/(52850 g x ºC)
- Cp = 0.65657521286 J/(g x ºC)
How do I calculate specific heat when no temperature is given?
That’s not possible. Q=mass × specific heat capacity x temperature is the formula, temperature cannot be removed from the equation.
How do I find the heat of a spice?
Look your spice up on the Scoville scale — it measures the pungency of spicy foods. A bell pepper is 0 on the scale. A mild jalapeno is about 3000, and a hot one is ~10000. Tabasco is around 30000, and a habanero can reach 350000.
If 200 grams of water is to be heated from 24.0 degrees to 100.0 degrees to make a cup of tea, how much heat must be added?
Q = C x m x dT Q = 4.18 x 0.2 x (100 – 24) Q = 73.112 J/g.C